For a hundred years young graduates have been told that “there’s a great future in plastics.” That exhortation continues to have currency today, thanks to the work of New Zealand born and educated scientist Alan MacDiarmid who was awarded the Nobel Prize in Chemistry for 2000 for his and his colleagues’ “discovery and development of electronically conductive polymers.” The Nobel Prize recognized advances that are seen to be the future of the technology fuelling the age of information. Alan, the model of a scientist, lives by the sign in his study: “I am a very lucky person and the harder I work the luckier I seem to be.”
A remarkable coincidence
In January of 1892, young Archie MacDiarmid had a country holiday at the farm of friends, the Rutherfords at Pungarehu on the Taranaki coast. In a letter home Archie mentioned that one of the Rutherford boys, “Ernest” had made 28 gallons of wine. Sixteen years later Ernest Rutherford was awarded the 1907 Nobel Prize in Chemistry.
It was to be 92 years before another New Zealand educated person was to win a Nobel Prize. It was Archie MacDiarmid’s son Alan.

Alan MacDiarmid receives the Nobel Prize for chemistry (2000) from His Majesty the King of Sweden. Image from Nobel Foundation website.
For centuries we have been used to electrical conduction in metals and also in liquids containing ions. For decades we have known that some electrical insulators can be made into conductors of electricity; for example in gases by creating an electrical discharge in them, and in some solid crystalline insulators by heating them to a high temperature. The discovery of organic polymers revolutionised materials science to give us plastics, amongst whose myriad uses is to safely insulate wires in electrical leads and devices. But the ability to make these polymers conduct electricity awaited the work of Alan MacDiarmid and his colleagues.
Family Catalyst
The MacDiarmids were a New Plymouth family, Archie was a marine engineer until he moved the family to Masterton in the Wairarapa about 1923, where he became head-engineer at Waingawa Freezing Works. Archie and Ruby had five children, the fifth of whom, Alan, was born in Masterton on the 14th of April 1927, in time for the Great Depression.
Shortly after winning the Nobel Prize Alan told one of his brothers how lucky he was to have been raised in a poor family which was also a close, loving family. Being poor made them self-reliant and conscious of the value of money whilst being close-knit taught them the importance of interpersonal relationships.
In Masterton the family lived on a hectare of land. Alan nearly died as a sickly toddler after the doctors had all but given up hope. Through the hard times of the depression, for four years of which Archie was unemployed, the family shared what they had with others less well-off whom Ruby invited for meals. The older children would call out to the younger ones “FHB”, to remind them that at such times the “family hold back”. Like most other children of the time, Alan went to primary school barefoot. The family bathed weekly, with the younger children using the bath water after the older children. Following a serious illness at about age nine, Alan was sent to recuperate for two or three months with an older sister who lived in Kerikeri.
As the depression eased Archie obtained work with a petrol company and the family moved to Lower Hutt.
These tough times imbued Alan with an unshakeable work ethic which he reflected on after receiving his Noble Prize:
“It is my home life while growing up through high school, which I consider to have been the single most important factor in any success which I may have had in life. As my parents always said, “…an ‘A’ grade in a class is not a sign of success.” Success is knowing that you have done your best and have exploited your God-given or gene-given abilities to the maximum extent. More than this, no one can do.”
Whilst at primary school he had an early morning milk round, delivering milk by bicycle for a local farmer. When he went on to Hutt Valley High School. in 1941, the early morning work was not possible. Instead, he delivered the “Evening Post” by bicycle after school. The close-knit family went on many picnics and hikes in the tracks of the Hutt Valley, instilling in Alan a love of the outdoors.
The Boy Chemist
His interest in chemistry was inspired at about age ten when he found one of his father’s old chemistry textbooks dating back to the late 1800s. This aroused a burning curiosity. He cycled to the Lower Hutt public library and on the right hand side on the bottom shelf of the new book section of the Children’s section he found a book with a blue cover “The Boy Chemist.” Alan kept it over a year, through continual borrowing. The foreword of this 1924 book, by the Reverend Archie Collins, stated:
“If you make the experiments in order, by the time you reach the tenth chapter you will have taken a fairly good course in Chemistry, and that will serve you well for all time.”

Alan at Hutt Valley High School.
His sister Alice recalls the wartime when fireworks for Guy Fawkes night were non-existent. Alan used his paper-round earnings to purchase chemicals and make his own fireworks. The family’s Guy Fawkes night, the only one in the district, went off with a bang.
At Hutt Valley High School Alan was always near the top of their best class. The Headmaster reported him as quiet, well mannered, helpful and a very good “type” of boy who took a prominent part in the outside life of the school. This included service in the wartime Air Training Corps. In 1943 he passed the University of New Zealand’s University Entrance Exam and its Medical Preliminary Exam.
Alan had to leave high school at age 16, after just three years, when his father retired on a very small pension and shifted to Kerikeri. Alan stayed on in Wellington, and from the age of 17 he supported himself. In 1944 he took a part-time, low-paying, job as lab boy in the Chemistry Department at Victoria University College.
Victoria was then a small university college with just 1200 students and only two staff in the Chemistry Department. Alan’s duties included washing dirty glassware, sweeping floors, refilling chemical bottles and preparing chemicals for the Stage 1 lecturer, “Bobbie” Munro, an inorganic chemist. On one occasion Alan was asked to prepare some S4N4-, which he did. The beauty of bright orange crystals was to have a profound effect on his future. He later explained his early inspiration for the research that would lead to a Nobel Prize: “It really stems from the fact that I like color. I like pretty things.”
Alan took two part-time courses, chemistry and mathematics, and remained a part-time student through his BSc and MSc degrees. To help make ends meet he became the live-in janitor at Weir House, the university hostel for men. He later recalled that that period was one of the most enjoyable and maturing times of his life. He made many good friends amongst the other ninety residents and still keeps in close contact with some.
After completing his BSc degree in 1947, he was appointed demonstrator in the undergraduate laboratories. One undergraduate of the time, Arthur Williamson, later recalled Alan mixing up a solution, which he then inadvertently placed in the sun. It exploded. For his MSc degree Alan asked Associate Professor Munro if he could study some of the chemistry of the golden crystals. This resulted in his first published paper, which appeared as a letter in that most prestigious of scientific journals, Nature.
 Alan graduated in 1951 with First Class Honours in Chemistry. He had failed to gain a scholarship to Cambridge University but was awarded a Fullbright Fellowship to do a PhD at the University of Wisconsin. Under Professor Norris Hall he majored in inorganic chemistry, studying the rate of exchange in C14-tagged complex metal cyanides. His stated plan was to return to New Zealand, should an appropriate position become available, and initiate the new field of using radio-active tracers in inorganic chemistry.
Alan graduated in 1951 with First Class Honours in Chemistry. He had failed to gain a scholarship to Cambridge University but was awarded a Fullbright Fellowship to do a PhD at the University of Wisconsin. Under Professor Norris Hall he majored in inorganic chemistry, studying the rate of exchange in C14-tagged complex metal cyanides. His stated plan was to return to New Zealand, should an appropriate position become available, and initiate the new field of using radio-active tracers in inorganic chemistry.
Alan became the President of the International Club, the largest student group on the Wisconsin campus. At a club dance he met his future wife, Marian Mathieu, they married in the chapel of his college, Sidney Sussex. He was belatedly awarded a New Zealand Shell Graduate Scholarship to study silicon hydrides at Cambridge University under H J Emeleus. After a brief appointment at the University of St Andrews, Scotland, Alan joined the faculty of the Chemistry Department at the University of Pennsylvania, Philadelphia, where he has remained. Marian died in 1990 after 36 years of marriage, his partner since 1991 has been Gayl Gentile.
Science and serendipity
Alan’s background at Victoria University College played a crucial role in the next piece of serendipity. Alan Heeger, a physicist at Pennyslvania, mentioned to him that in a recent paper (SN)x was reported as a highly conductive material. Because Alan had made its precursor S4N4, Alan Seeger asked him to prepare (SN)x as golden crystals. They co-authored many papers on this conducting polymer.
The serendipity of science took another hand in Alan’s future. In 1975, while a visiting Professor at Kyoto University, lecturing on molecular silicon compounds, Alan visited Tokyo Institute of Technology to describe his work on (SN)x. After the talk he had green tea with Hideki Shirakawa who showed him silvery (CH)x, the sure sign that a material was conducting electricity. This was the product of a mistake by a recently arrived foreign student whom Shirakawa had asked to extend some work he (Shirakawa) had been doing. The work involved polymerising ordinary acetylene welding gas using a Ziegler-Natta catalyst. Misunderstanding the Japanese word for millimolar concentration, the student used a catalyst concentration 1000 times stronger than Shirakawa had instructed and produced not the black-brown powder Shirakawa expected but silvery-pinkish jelly-like lumps.
Alan invited Shirakawa to join him for a year at Pennsylvania. There they attempted to make very pure silvery polyacetylene, in order to increase its electrical conductivity, but found instead that it decreased. However Alan had increased the conductivity of golden (SN)x by doping it with bromine, and the two scientists found that the same technique worked with (CH)x increasing the conductivity many millions fold.

Nobel chemistry: class of 2000: Alan G. MacDiarmid, Hideki Shirakawa and Alan J. Heeger. Image from Nobel Foundation website.
At 3.00am on October 14th, 2000, Alan phoned his sister to say that he, Hideki Shirakawa and Alan Heegar had been awarded the 2000 Nobel Prize in Chemistry.
Two days later he rang again to invite his whole family to the ceremony on Dec 10th, the anniversary of Nobel’s death. In all he paid for 22 family members to travel to see him presented with the gold medal by the King of Sweden at a sumptuous ceremony in the Stockholm Concert Hall.
Research, Dedication, Application
Alan is the epitome of a research scientist embodying total dedication to his subject. He keeps a writing pad beside his bed to jot down ideas that come in his most productive thinking times such as dozing or when in the shower. But science is people, singular to collective: discussing ideas with colleagues; the close relation between supervisor and research student; each problem solved raising another half dozen; and the continuous discussion that makes it difficult to recall who produced the crucial step when filing for a patent. Alan modestly subsumes his own contribution beneath his dedicated enthusiasm for the work:
“We were fascinated by – we were excited about the science. In other words, we lived it, breathed it, slept it, dreamed it; complete immersion. And at first people didn’t necessarily believe what we were saying, but that slowly changed.
“If you go to Las Vegas or Atlantic City and put a quarter in the slot machine, a vague thought goes through your mind that maybe you might hit the jackpot. Similarly, if you go into a lab and play around with new things, the thought vaguely goes through your mind that maybe you might hit a scientific jackpot. But it’s not something you really consider seriously.”

Alan in his U. Penn classroom
Conductive plastics have been developed into many useful applications such as lightweight electromagnetic shields and ‘smart’ windows that can vary the amount of light they allow to pass. Now second generation devices have surfaced: transistors, light-emitting diodes, and lasers. One beauty of plastics is that they are low cost and can be prepared as films. It is quite likely that electroluminescent films papering walls will provide the power-saving illumination of the near future. The innovation already appears in higher quality photographic films and cell phone screens.
Single molecule electrical circuits connected by conducting polymers may be the electronics of the future; playing a major role in the development of new electronic devices. The application of the research into plastic circuitry is at the forefront of nanotech innovation and on the brink of transforming information technology. With the potential to reduce the size of componentry used in computers to the atomic scale, the speed of computer computations and the size of computer memory, could be increased by millions of times. As the press release accompanying Alan’s Nobel citation reads, “a computer corresponding to what we now carry around in our bags would suddenly fit inside a watch.”

nanotech: malleable and conductive plastic
Alan loves teaching, being stimulated by young minds. Now in his seventies, he is still fully engaged in teaching, including, at his request, a first-year chemistry class.
“In our own research we have had chemists, and physicists working closely together. This brings home the enormous importance of discussion in research. You can be the most brilliant scientist in all the world; put you on a desert island with the very best scientific equipment and the very best library and you’ll do uninteresting research. You must have interaction. You must have discussion.
“This award to Alan Heeger and to Hideki Shirakawa and me is recognition of the work that we have done–each of us individually and together. We have been fortunate to have first-class students – undergraduate, graduate and post-doctoral – in our groups. And I always say research coming from a given group can never be better than the people carrying it out. The prize is recognition of their work.
“Let’s say that I had some brilliant crazy idea to turn lead into gold and I asked a student who is not very good. He will say, ‘Well I tried to turn lead into gold and it didn’t work. I tried it six times and it didn’t work.’ Then I might turn to a very good student who might come back after a week and say, “Well, I’ve been able to turn a little bit of lead into gold.” So what’s the difference? If you have very good people working, not for you but with you, then the chances of finding very important, critical, unexpected things are pretty high.”
Alan always retained his ties with New Zealand, with family, friends and scientists, and he regularly holidays here. For some years, he has been collaborating with colleagues at the Industrial Research Laboratories and at Victoria University of Wellington. The latter gave him an honorary doctorate in 1999 and in 2001 created the Alan MacDiarmid Chair in Physical Chemistry. In 2000 the Royal Society of New Zealand awarded him its top honour, the Rutherford Medal. In 2002 he became a Member of the Order of New Zealand, which is limited to 20 living people and is the highest honour that his home country awards.

Alan MacDiarmid’s Nobel diploma. Image from the Nobel Foundation website.
New Zealand has now had Nobel Prizes in Chemistry awarded to two of its sons who were born, bred, educated and started their research here: Ernest Rutherford and Alan MacDiarmid. It is indeed a lucky country that can lay claim to awardees with such fine individual qualities as these two – may the land continue to conduct such currents.
Post script:
Alan MacDiarmid died after a fall on 7 February 2007, at his home in Philadelphia, aged 79. Alan was preparing to travel to New Zealand, where he was scheduled to give a lecture on non-polluting renewable energy.
References:
[All accessed October 2001]
Alan MacDiarmid’s U. Penn homepage.
Official Nobel Prize page with an excellent and educative illustrated explanation of the science behind the award.
Presentation from MacDiarmid to the Royal Society of New Zealand,
University of Pennsylvania press conference lauding MacDiarmid.
MacDiarmid’s first alma mater Victoria University celebrates his achievements, including the establishment of a chair in his honour.
U.Penn almanac interview with MacDiarmid including why he left New Zealand.
BBC article on the Nobel Award and the innovation the research represents.
Physical Review coverage of the Nobel award and why the “electricity through plastic” research is so important.
“It’s not just for tupperware anymore” – Business 2.0 article on the huge potential application of the science.
US Department of Energy article on MacDiarmid and plastic batteries.
USA Today article recognises the Nobel winners as “information age pioneers.”
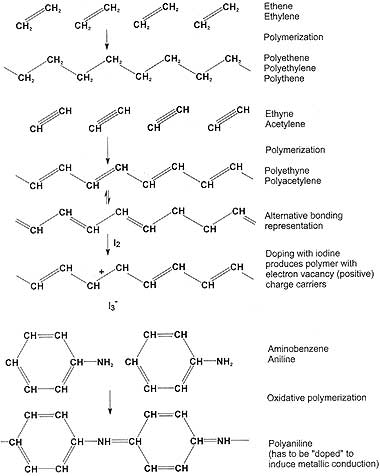
Representation of the doping processes that achieve conducting organic polymers. From the New Zealand Science Review Vol 58 (1) 2001 pp 14-17.
STORY BY JOHN CAMPBELL
Dr. John Campbell, author of Rutherford: Scientist Supreme, and www.rutherford.org.nz, teaches physics at the University of Canterbury.












