The creator of modern atomic physics and forerunner of the nuclear age, Rutherford was one of the greatest scientists of the 20th century. He was awarded the Nobel Prize in Chemistry in 1908 and a baronetcy in 1931, choosing the title Baron Rutherford of Nelson. In the words of Einstein, he was “a second Newton”, the man who “tunneled into the very material of God”: inventor, experimenter and Nelson farm boy.
Rutherford’s strengths as a scientist are legion. A prolific, practical inventor and scientific theorist, his ideas were based on rigorous experimentation. He was one of the original “demo or die” scientists, turning conjecture into fact. He attributed his willingness to experiment and find unorthodox solutions to his hardscrabble background in rural New Zealand: “We don’t have the money, so we have to think”.
Three Discoveries
Ernest Rutherford’s three major discoveries shaped modern science, created nuclear physics and changed the way that we envisage the structure of the atom.
Rutherford’s first discovery was that elements are not immutable, but can change their structure naturally, from heavy elements to slightly lighter. This led to him being awarded the Nobel Prize for Chemistry in 1908, at the age of 37, for his work on the transmutation of elements and the chemistry of radioactive material.
His second discovery, the nuclear model of the atom, became the basis for how we see the atom today: a tiny nucleus surrounded by orbiting electrons.
He built on this discovery for his third great achievement, the splitting of the atom, making him, as John Campbell says, in his biography of Rutherford in The Dictionary of New Zealand Biography, “the world’s first successful alchemist”.
Counting The Beats
Ernest Rutherford was born in Brightwater, near Nelson, New Zealand, in 1871. He was the fourth child and second son of 12 children, to James Rutherford, a mechanic, wheelwright, engineer, flax-miller and farmer and his wife, Martha Thompson, a school teacher before her marriage. Both parents were keen that their children gain an education, and were supporters of the small local schools where Rutherford and his brothers and sisters began their schooling. Martha ensured the Rutherford children completed their homework with the dictum, “All knowledge is power.”
From an early age Rutherford was distinguished at school for his arithmetical abilities and his scientific curiosity. Both qualities were encouraged by his early teachers, Harry Ladley at Foxhill and Jacob Reynolds at Havelock School. Reynolds gave extra lessons in Latin and algebra for children of above average ability, including brothers Ernest and Jim Rutherford. Rutherford’s early education, from school, from his family and from exploring the local farms and countryside with his siblings, awakened his interest in science and developed the the keen observational skills that are essential for the scientific mind. A school science text-book told of a method for determining the distance of an enemy’s cannon, a method which Rutherford adapted to local surroundings during an electrical storm at Foxhill. As Eugene Grayland recounts in Famous New Zealanders in a reconstruction of an anecdote from Rutherfrod’s childhood:
“James Rutherford, who had got out of bed to check on the storm, was surprised, more so when he heard his son talking to himself softly.”
‘Ernest, what’s up, my boy?’ he called out.
‘I’m counting,’ the boy called back.
‘Counting?’
There was a rumble of thunder which shook the house.
‘Yes. If you count the seconds between the flash and the thunder clap and allow 1,200 feet for each second for the sound to travel, you can tell how close you are to the storm centre.’ “

Rutherford Family, early 1883
Left to Right: Charles (8 yrs), Ernest (11 yrs), Florence (6 yrs), Martha (40 yrs), James (10 yrs), Herbert (9 yrs)
Appropriately, Rutherford’s first recorded experiment was a cannon constructed from the brass tube of a hat-peg with a marble for a ball and a dose of gunpowder to ignite the device. It was not the best example of Rutherford’s experimental savvy, the resulting explosion failing to deliver the marble to the target twenty metres away, but succeeding in destroying the cannon.
The Rutherfords were a close-knit family, gathering around the piano to sing songs; forging a life with few amenities in the isolated and rugged landscape. Though two of the brothers drowned in a childhood accident and another died as an infant, the life of the Rutherford siblings was filled with the curiosity-satiating distractions of growing up in the New Zealand outdoors. There were the stimuli of farm-life: poaching eggs from bird’s nests, orchard raiding, swimming in the Wai-iti river, shooting Kereru pigeons fat from feeding on berries, calculating the level for storage ponds at the flax-mill.
Earning enough to feed the family was a struggle for James Rutherford at times. He ran a farm and flax-mill at Foxhill, and another at Pelorus when the family moved there, in 1883. In 1885 he turned to saw-milling, manufacturing railway sleepers for the Government. However due to an economic downturn his contract was cancelled (while he was recovering from an accident which left him with five broken ribs) and he had to leave the family to look for new opportunities in the North Island. He founded a steam driven flax-mill in Pungarehu, Taranaki, employing twenty people, where he moved the family in 1888.
In the school holidays Rutherford busied himself with farm chores, helping out on the farm or at the mill. He had distinguished himself from his earliest days at school, but it took two attempts for him to win an education board scholarship and follow his older brother, George, to Nelson College. For children of less-than-wealthy parents a scholarship was one of the few options available with which to obtain further learning. Rutherford attended Nelson College as a boarder for three years, and came under the tuition of William Littlejohn, who taught him mathematics and elementary science.
He topped his class in every subject in his final year and, after sitting the exam twice, won one of ten nationwide Junior Scholarships. In his final year he was also head boy, dux, and was a forward in the rugby First XV.
In 1890, he enrolled at Canterbury College, University of New Zealand (now The University of Canterbury). At Canterbury College he continued to play rugby and took part in the student Dialectic Society (a debating club) and the Science Society.
Early Experiments
In 1892, Rutherford completed a Bachelor of Arts degree from Canterbury College and won the only available Senior Scholarship for mathematics. This made it possible for him to return to university for an Honours year, completing a Master of Arts with double First Class Honours in Mathematics and Physics.
At Canterbury he was taught by Professor Alexander Bickerton, whose “genuine enthusiasm for science gave a stimulus to me to start investigations of my own”, as Rutherford would credit later. It was in 1893 that his talent for original experimentation and research began to manifest itself: a penchant for creating innovative experiments to solve problems. The findings in his first year’s research were based on his invention of a machine that could measure time differences of up to hundred-thousandth of a second. With this device he demonstrated that it was possible for iron to be magnetized by high frequency currents.
In 1894, Rutherford completed a Bachelor of Science in Geology and Chemistry and in 1895 was awarded an Exhibition of 1851 Science Research Scholarship (but only after the top-ranked candidate withdrew). He elected to work as a research student at the Cavendish Laboratory, University of Cambridge, under Professor J.J. Thomson. The Professor was studying the conduction of electricity in rarefied gases, which led to his 1897 discovery of the electron. This was the first object to be discovered that was smaller than an atom.
At Nelson College and Canterbury College, fostered by Bickerton, Rutherfordhad been no more than an excellent student. With his move to Cambridge, on a scholarship designed to benefit young graduates from the outposts of Empire, his gifts were to be fully recognised (Rutherford was amongst the first “foreign” students to be admitted to Cambridge, without going through the undergraduate system). Family anecdote recalls that Rutherford was working on the farm when he received news of the scholarship: “That’s the last potato I will ever dig” he remarked.
Cambridge, McGill, Manchester
Once in Cambridge, he amazed Thomson with his enthusiasm, tenacity and fresh approach. As Campbell has written, Rutherford went to Cambridge with a reputation as an innovator and inventor, and distinguished himself in several fields, initially by divining the electrical properties of solids and then using wireless waves as a method of signalling:
“Rutherford was encouraged in his work by Sir Robert Ball, who had been scientific adviser to the body maintaining lighthouses on the Irish coastline; he wished to solve the difficult problem of a ship’s inability to detect a lighthouse in fog. Sensing fame and fortune, Rutherford increased the sensitivity of his apparatus until he could detect electromagnetic waves over a distance of several hundred metres. Thomson […] quickly realised that Rutherford was a researcher of exceptional ability and invited him to join in a study of the electrical conduction of gases. The commercial development of
wireless technology was thus left for Guglielmo Marconi.”
Rutherford’s advances in the study of radioactive atoms (most notably discovering that two different emissions, named alpha and beta rays, emanate from radioactive atoms) and his genius for experimentation secured his reputation, even compared to his brilliant mentor Thomson. In 1898, at the age of 27, he moved to McGill University in Montreal, where he held the position of Professor of Physics.
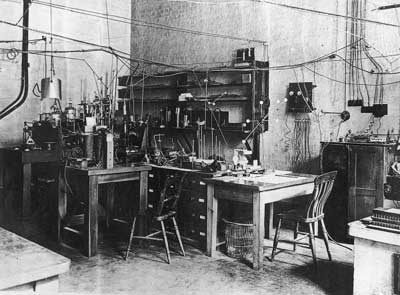
c. 1926 The apparatus used by Ernest Rutherford in his atom-splitting experiments, set up on a small table in the centre of his Cambrige University research room – Caverndish Laboratory.
From the book ‘Century’ by Phaidon published 1999 Page 242
The McGill years, from 1898 to 1907, were significant for two major developments. Firstly, Rutherford was finally on a secure enough financial footing to marry his long-time fiancé, Mary Georgina Newton. She was the daughter of Mary Newton, who was Rutherford’s landlady in Christchurch, prominent in the movement which saw the women of New Zealand granted the vote in 1893. Rutherford and Mary were married in 1900 in Christchurch. Their only child, Eileen, was born in 1901.
Secondly, it was at McGill that Rutherford made the first of his three major discoveries. Assisted by chemist Frederick Soddy, he unravelled the mysteries of radioactive atoms. He rejected the popularly accepted belief that elements were immutable, which the word atom itself implies. It derives from the Greek “tomos“, meaning to cut, and “a” meaning not; therefore an atom is something unsplittable. Rutherford demonstrated that in fact some heavy atoms spontaneously decay into slightly lighter, and chemically different, atoms. His book on this subject, Radioactivity, was published in 1904, followed by others in 1906 and 1930. It was this discovery and his work on the chemistry of radioactive materials, that led to him being awarded the Nobel Prize in Chemistry in 1908 for his “investigations into the disintegration of the elements, and the chemistry of radioactive substances.”
While at McGill, Rutherford also developed a range of devices including one for measuring vibrations caused by streetcars and another for trains to signal to stations using wireless telegraphy. Much of the apparatus he developed is today housed at McGill’s Rutherford Museum.
In 1907, at the age of 36, Rutherford was lured back to England to become Professor of Physics at Manchester University.
Dissecting The Atom
 In 1907, Rutherford began a debate with physicist Antoine Becquerel on how alpha particles, reacted when they were ejected from radioactive material. Discovering that they tended to bounce off air molecules, he surmised that there had to be something at the centre of atoms to deflect them. He tested his assumptions by bouncing alpha particles off a sheet of gold leaf and determined that the most powerful part of an atom was a very small, heavy, core at its centre, an electrical charge concentrated at a point – the nucleus. This was surrounded by a cloud of electrons made up of an opposing electrical charge.
In 1907, Rutherford began a debate with physicist Antoine Becquerel on how alpha particles, reacted when they were ejected from radioactive material. Discovering that they tended to bounce off air molecules, he surmised that there had to be something at the centre of atoms to deflect them. He tested his assumptions by bouncing alpha particles off a sheet of gold leaf and determined that the most powerful part of an atom was a very small, heavy, core at its centre, an electrical charge concentrated at a point – the nucleus. This was surrounded by a cloud of electrons made up of an opposing electrical charge.
This concept of opposite charges which, as David Eliot Brody and Arnold R Brody noted, “marks the beginning of the modern understanding of the structure of the atom”, was Rutherford’s second great discovery. As Campbell says, “the nuclear model of the atom had been born”.
This orbiting model was the most revolutionary idea of Rutherford’s career, as Nigel Costley, writing in the Sunday Star Times, relates:
“Prior to Rutherford the best model of the atom was J.J. Thomson’s plum pudding which pictured it in a thin cloud of positive charges, with electrons dotted amongst it, like so many raisins in a plum pudding.”
“Rutherford’s experiments showed the pudding idea was wrong, replacing it with a solar system model. An incredibly dense positively charged nucleus lay at the centre which was tiny compared to the whole atom; like a postage stamp in a football field.”
“Through his studies of radioactivity in the 1890s, he discovered alpha particles which became, in his skilled and determined hands, the chief weapon in prising out the secrets of the sub-atomic world. These particles were known to be 7400 [closer to 7273] times heavier than electrons and when he fired them in huge numbers at a strip of very thin gold foil, it was expected they would effortlessly pass straight through.”
“However a surprisingly high number were deflected […]. Rutherford was astonished: “It was as if you fired a 15-inch shell at a sheet of tissue paper and it came back to hit you.” From these deflections he was able to calculate the size of the nucleus.”
During World War I, Rutherford worked on acoustic methods of detecting submarines and developed several new technologies. He then drew on a lifetime’s strengths in practical experimentation for the third great breakthrough of his career.
Radioactivity had shown that some atoms spontaneously split, but in 1917 (reported 1919) the committed alchemist

Hans Geiger and Ernest Rutherford with apparatus for counting alpha particles, Manchester, 1912
Permission: www.corbis.com
Rutherford, as McLauchlan writes, “detected the transmutation of one elementary material, nitrogen, into another, oxygen, which was induced artificially when the nitrogen atom was bombarded by the natural alpha articles of radium.” Rutherford was, as he describes the process himself with typical understatement, “playing with marbles.” He was using alpha particles to eject protons from materials containing hydrogen when he found the same thing happened in nitrogen (which doesn’t contain hydrogen). But more importantly the proton came out at higher energy than it could have received by collision.
In that year Rutherford had written to Niels Bohr that, “… I am also trying to break up the atom by this method … Regard this as private.”
Effectively Rutherford had “broken up” or split the atom. With this experiment, he was the first human to create a “nuclear reaction”, though a weak one. From Rutherford’s first discovery onwards he had swept away accepted models of the stable atom, altered the course of modern science and made possible the development of nuclear physics. Once more Rutherford’s demonstrations had changed the way we viewed and conceived of the world, breaking through the gross world of matter into the subtle world of atoms.
Return to Cambridge
Rutherford returned to Cambridge’s Cavendish Laboratory as Director, in 1919, and became well known for a personality to match his achievements, mentoring and directing others towards great discoveries. Prof. S. Devons in A Hundred Years and More of Cambridge Physics: Rutherford’s Laboratory recounts:
“Cambridge, and the Cavendish Laboratory especially, was an established, renowned centre of science. In the early 1930’s its lustrous reputation was as high as ever. These years were indeed the “golden age” of the Cavendish …”
“His influence there seemed a wholly natural phenomenon. Benevolent guidance, leadership and intellectual authority flowed from him, and loyalty was returned. One would no more question his influence on those around him than one would that of the sun on the satellite planets. Rutherford, the Cavendish Professor, was the centre of light and warmth and life. It was the natural order of things. “
Michael Kelly (one of the many New Zealanders who followed in Rutherford’s wake to study physics at Cambridge and now a world leader in the field of solid-state physics) has said that because of his tenacity, Rutherford was popularly known as “crocodile”. This was because, as well as connoting the father of the family, a crocodile can never see its tail. “It always looks forward and that’s how he was … he would make bold imaginative leaps at what might be going on before setting up the experiments to check it. There are other people who work in much more formalistic ways, but he had an idea on the main chance.”
Rutherford set high standards of research at Cambridge. Michael Kelly again: “Rutherford’s style of doing research set the tone for much of the experimental work done at Cambridge. His was very much a sealing wax and cotton style – ‘let’s have a go’.”
A Genius For Astonishment
In Richard Rhodes’ book, The Making of the Atomic Bomb, one of Rutherford’s protegees, James Chadwick, summed up his mentor as follows: “Rutherford’s ultimate distinction was ‘his genius to be astonished’.”
This key to Rutherford’s thought and approach reflects Rutherford’s background as much as his personality: growing up in the rough and ready New Zealand backblocks, he was relatively free of the social constraints and acceptance of intellectual assumptions that marked the more genteel culture of British physicists. What Rhodes has called the “braiding of country-boy acuity with a profound frontier innocence” made Rutherford free of preconceptions and independent of accepted theories and assumptions, leading to his originality as a thinker and experimenter. A fellow student in his early days at Cambridge noted: “We’ve got a rabbit here from the Antipodes, and he’s burrowing mighty deeply.”
In his study of Rutherford’s life, Nigel Costley reports that:
“…you could tell when work was going well in Rutherford’s laboratory: he strode about singing a spirited rendition of “Onward Christian Soldiers.” His character, full of hearty good humour interspersed with imperious commands, was more that of a boisterous colonial farmer than the world’s leading scholar. Yet by virtue of his forceful personality and an intuition for picking the right experiment, he was a revolutionary ….
“There was a paradox in this combination of an elderly conservative gentleman of the “old school” and the proponent, nay the discoverer, of the latest word in this most modern field of knowledge: atomic and subatomic physics…it was all part of the scene: Cambridge, the Cavendish and Rutherford alike; traditional forms and radical ideas; an enduring, time-beaten outer shell containing and protecting the vital, quickening activity within.”
Simplicity was the key to Rutherford, says Devons:
“There was an extraordinary transparent honesty and a deceptive simplicity
about the clear distinction between fact and theory (opinion). He was impatiently hostile to any attempt to obscure or to conceal or to complicate unnecessarily…it was the remarkable combination of a most powerful imagination counterbalanced by a sense of utter honesty that was most impressive and mystifying.”“Rutherford’s emphasis on simplicity is proverbial: (“I’m a simple man myself…”). Simple ideas and simple apparatus, but powerful, conclusive results; simple, unpretentious appearances, but striking inferences: these were the Cavendish trademarks.”
Rutherford was a man of great energy and persistence, a keen golfer and motorist, and a mentor for young science students in Britain, especially New Zealanders.
The Great Mentor
James Chadwick, who won the Nobel Prize for Physics in 1935 for discovering the neutron (a particle first predicted to exist by Rutherford in 1920), was one of a number of scientists who studied under Rutherford and achieved lasting fame. Another notable young colleague was Niels Bohr, who won his own Nobel Prize (for Physics in 1922) for placing the electrons in stable orbits around Rutherford’s nucleus and thus explaining the origin of light emitted by hydrogen atoms. While at Manchester, Rutherford’s assistant was Hans Geiger, and the 1907 Rutherford-Geiger detector was improved in 1928 to become the Geiger-Muller tube we know today for measuring radiation. Robert Oppenheimer, later to be known as the “father of the atomic bomb” for his leading role in developing the bomb in the Los Alamos Laboratory during the Second World War, also studied at Cavendish under Rutherford. John Cockroft and Ernest Walton were driven by Rutherford to construct the first high energy accelerator and were the first to use it to split the nucleus by entirely artificial means.
The National Hero
 Rutherford won a series of honours for his work, including the 1908 Nobel Prize for Chemistry, and 21 honorary degrees. He has featured on the stamps of New Zealand, Sweden, Russia and Canada. In 1931, at the age of 59, he was named Baron Rutherford of Nelson, choosing as his coat of arms a design that included a kiwi and a Maori warrior. He remained proud of his New Zealand origins and his family: on being awarded his baronetcy, he sent a telegram to his mother: “Now Lord Rutherford. More your honour than mine. Ernest.”
Rutherford won a series of honours for his work, including the 1908 Nobel Prize for Chemistry, and 21 honorary degrees. He has featured on the stamps of New Zealand, Sweden, Russia and Canada. In 1931, at the age of 59, he was named Baron Rutherford of Nelson, choosing as his coat of arms a design that included a kiwi and a Maori warrior. He remained proud of his New Zealand origins and his family: on being awarded his baronetcy, he sent a telegram to his mother: “Now Lord Rutherford. More your honour than mine. Ernest.”
However the baronetcy was awarded at a sad time in his life: The Rutherford’s only daughter, Eileen had died eight days before, just nine days after the birth of her fourth child.
On visits Rutherford made back to New Zealand, he was a celebrated figure. He came home for the last time in 1925, for six weeks, to see family and give lectures. In Auckland he stated, “I have always been very proud of the fact that I am a New Zealander.” Described by reporters as “an imposing figure, tall, well-built and with bright blue eyes”, Campbell chronicles how Rutherford was hailed as a national hero, lectured to packed halls and called for the Government to protect New Zealand’s natural heritage. He also called for an institute to be set up in which New Zealand scientists could carry out research that would benefit farmers: this assisted in the establishment of the Department of Scientific and Industrial Research in 1926.
He was still a seemingly healthy, vigorous man, when in 1937, he entered hospital for a minor hernia operation after straining himself cutting down some trees. Within a few hours of the operation it was clear his intestines were not working and they never worked again. Four days later, he suddenly said to his wife from his sickbed, “I want you to leave one hundred pounds to Nelson College. You can see to it.” Then he added more loudly, “Remember, a hundred to Nelson College.” He hardly spoke after that according to his wife, and at the early age of sixty-six, Rutherford died, on 19th October, 1937. His ashes were interred at Westminster Abbey near the tombs of Isaac Newton and Lord Kelvin. His medals were gifted to Canterbury College, now University of Canterbury. In 1992, his image was placed on the new New Zealand $100 note.
The Quotable Rutherford
On his vocation:
“All science is either physics or stamp collecting.”
“The more physics you have the less engineering you need.”
On New Zealand innovation:
“We don’t have the money, so we have to think.”
On his experiments that resulted in his discovery of the model of the atom (the scattering experiments):
“It was as if you fired a 15-inch shell at a sheet of tissue paper and it came back to hit you.”
On splitting the atom:
“I have broken the machine and touched the ghost of matter.”
On a self-important person:
“That man is an Euclidian point: position without substance.”
For key references on Rutherford’s life and achievements:
Books
Campbell, J. (1996) “Ernest Rutherford”, The Dictionary of New Zealand Biography, Volume Three, 1901 – 1920. Auckland University Press/ Department of Internal Affairs.
Campbell, J. (1996) Rutherford’s Ancestors. Christchurch, AAS Publications.
Campbell, J. (1999) Scientist Supreme. Christchurch, AAS Publications.
Grayland, E. (1967) Famous New Zealanders. Christchurch, Whitcombe and Tombs Limited.
McLauchlan, G. ed. (1995) Bateman New Zealand Encyclopedia 4th edition. David Bateman Ltd.
Oliphant, M. (1972) Rutherford – Recollections of The Cambridge Days. Elsevier, (a personal account of Rutherford’s time at Cambridge).
For more about his scientific and other discoveries:
Rhodes, R. (1986) The Making of the Atomic Bomb. Simon & Schuster.
Riley, R. (1995) Kiwi Ingenuity: a Book of New Zealand Ideas and Inventions. AIT Press.
Brody, D.E. & Brody, A.R. (1997) The Science Class You Wish You Had. Allen and Unwin.
Articles:
Waterson, S. (1999) “Ernest Rutherford – The Most Influential People in the South Pacific”, Time magazine, October 25.
Easton, B. (1997) “Kelly’s Crystal Career”, The New Zealand Listener, June 21.
Costley, N. (1999) “‘Crocodile’ Launched World Into Atomic Age”, Sunday Star Times, October 17.
For a record of the life and career of Rutherford, as shown by the 36 medals he was awarded during his life:
Stocker, M. (1999) Golden Atoms: The Ernest Rutherford Medals. Canterbury University Press.
Web References:
For the comprehensive Rutherford page see:
Rutherford biographer Dr. John Campbell’s Rutherford.
<http://www.rutherford.org.nz>
[Accessed September 2001].
For a limited account of Rutherford’s early years:
Cox, I and Wittal, M. Rutherford, The Early Years.
<http://www.nelson.planet.org.nz/~richmond/rutherfd/cover.htm>
[Accessed November 1999].
For an account of Rutherford’s Cambridge years, see:
Devons, S. “Rutherford’s Laboratory”, A Hundred Years and More of Cambridge Physics. Cavendish Laboratory / Cambridge University Physics Society.
<http://www.phy.cam.ac.uk/cavendish/history/years/rutherford.asp>
[Accessed November 1999].
For more about his scientific achievements you can also look at:
A Science Odyssey: People and Discoveries. WGBH
<http://www.pbs.org/wgbh/aso/databank/entries/bpruth.html>
[Accessed November 1999].
“The Nobel Prize for Chemistry 1908 : ERNEST RUTHERFORD Biography”. The Electronic Nobel Museum. The Nobel Foundation.
<http://www.nobel.se/laureates/chemistry-1908-1-bio.html>
[Accessed November 1999].
Newsgroups discussion archive
<http://nz.com/NZ/People/Rutherford.html>
[Accessed November 1999].
“Chemistry & New Zealand: Who was Ernest Rutherford?”. Kiwi Web.
<http://www.chemistry.co.nz/ernest_rutherford.htm>
[Accessed November 1999].

Rutherford:
A play by Stuart Hoar
Directed by Susan Wilson
Supported by Creative New Zealand
World Premiere
Circa Theatre
New Zealand Festival 2000
Saturday 4 March 2000.
“From Nelson farm boy to father of the nuclear age – who was Rutherford? What becomes of a man who tries to tunnel into the very material of God? An exciting theatrical exploration of the life and work of revolutionary New Zealand physicist Ernest (Lord) Rutherford. The play focuses on the enigma that was Rutherford, follows his obsession with science and probes his personal relationships with his wife Mary, daughter Eileen, and friend and colleague, the Russian, Kapitza.
“Circa, in association with Factory School of English, Film and Theatre, Victoria University of Wellington, is proud to present the world premiere of this intriguing new work from leading New Zealand playwright, Stuart Hoar. “Rutherford” promises a startling and engrossing journey of discovery in the fascinating experiment that was the life of Ernest Rutherford.”
The Rutherford Documentary:
The Rutherford documentary is based on John Campbell’s book “Rutherford Scientist Supreme” and was written, directed, editored, and co-produced by Gillian Ashurst. The co-producers were John Campbell and Gillian Ashurst.
The documentary tells the story of Rutherford’s life and work in three one hour episodes. John Campbell was awarded a 2012 Education and Communication Award from the Canadian Nuclear Society for the documentary.
Purchase your DVD copy of the Rutherford documentary from John Campbell at Rutherford.org.nz.

















testing comments box on legend page carla
Stumbled upon NZEDGE when I was looking for information on Lord Rutherford (it still burns me that Clinton said that Fermi split the atom!). I was really surprised to find that so many heroes have come from New Zealand. Nancy Wake, Keith Park, Charles Upham and so many more - I never learned anything of these people when I was at school. Thanks to the NZEDGE my identity as a Kiwi is stronger than ever before. Cheers from NYC, John. Equity Analyst New York, USA
I enjoyed the material on Ernest Rutherford. Could you please provide a reference for the quote of a Michael Kelly to the effect that Rutherford was called the "Old Croc". You might mention that the protons discovered by Rutherford are a potent form of radiation which is being utilized with great effect in the treatment of patients with many types of cancer. Thank you, Herman Suit, MD, D Phil [Oxon] Professor Harvard Medical School Editor replies: Michael Kelly's quote that Ernest Rutherford was known as the Old Croc is from The New Zealand Listener, June 21, 1997, p32. The article runs: "Michael Kelly is among the world’s leading solid-state physicists. Now head of the electronic and electrical engineering departments at Surrey University, he mixes with Nobel prizewinners and has joined an elite bank of New Zealand scientists to be elected a fellow of the Royal Society Although it is tempting to say that he is walking in the footsteps of Rutherford. Kelly, 47, airily dismisses the suggestion. He does concede, however, that Rutherford paved the way for New Zealand scientists - himself included - who spent part of the careers studying at Cambridge. "Rutherford’s style of doing research set the style for much of the experimental work done at Cambridge," says Kelly. "His was very much a sealing wax and cotton style - ‘let’s have a go’ - and then he would make bold imaginative leaps at what might be going on before setting up the experiments to check it. There are other people who work in much more formalistic ways, but he had an idea on the main chance. There is, in one building at Cambridge, etched into the brickwork, a statue of a crocodile. Rutherford was known as the Old Croc, because a crocodile can never see its tail. It looks forward, and that’s how he was."" The following is taken from the website of the Cavendish Laboratory: "A striking feature of the old Cavendish site is the carving of a crocodile on the outer wall of the Mond Laboratory. The Laboratory was built in 1933 by the Royal Society for Kapitza to continue his work into intense magnetic fields. During the building work, those passing the lab were suprised to see a figure in a brown monk's habit busily chipping away at the brickwork behind a tarpaulin screen. This was Eric Gill who had been commissioned by Kapitza to carve both a plaque of Rutherford and this Crocodile - "The Crocodile" being Kapitza's pet name for Rutherford, either because of his fear of having his head bitten off by him, or because his voice could be relied upon to precede his visits, just like the crocodile's alarm clock in "Peter Pan". Regards, Editor. Herman Suit, Physician Boston, USA
"Great place to look up homework things for New Zealand kids! Thanks for the stuff on Lord Rutherford. I was sick of everyone calling him a British Physicist when he was born near Nelson!" Early Childhood Educator, Radio Copywriter and Mother Whakatane, New Zealand
He is arguably the greatest experimental scientist in history. His major achievements were 1 Explaining the nature of radioactivity 2 Providing a model for the structure of the atom 3 Transmutation of elements (the first successful alchemist?). In 1919 he converted nitrogen to oxygen by bombarding its atoms with energetic radiation. Other work was done on the age of the Earth, very high voltage transformers, submarine detection in World War 1 and electrical technology. In the Nazi regime of the 1930s he was influential in helping Jewish scientists escape and find refuge and work in other countries. In the matter of teachers who had a major influence on the young Rutherford, Jacob Reynolds at Havelock School encouraged the boy to study and sit for the Marlborough Scholarship which eventually sent him to Nelson College. He was successful at his second attempt at the age of 15. Ladley, at Foxhill School earlier, undoubtedly played a part in influencing the boy, but John Campbell attributes the major early influence to Reynolds. As you note, it is also apparent that his parents, as well as his siblings and companions [Matthews children] were significant in his education at this stage. At Nelson College it was William Littlejohn who took an interest in Ernest and helped him with his studies, and at Canterbury College he came under the tutelage of William Bickerton, a remarkable lecturer and populariser of science. Ross Alistair Gillanders
"I am a student in Auckland College and I am doing a research assignment on Ernest Rutherford at the moment and I really found your article very good. I came up with couple of questions I would like to know about Rutherford. 1. Why Rutherford did not stay in New Zealand to research? Why did he go overseas to research? What were his reasons to go overseas? 2. How is his success and himself shown right now to be remembered by the people? What are the reasons he is on the $100 note now." Ed’s reply: "Thanks for writing. Rutherford's reasons for going overseas would have simply related to the fact that the international scientific community was there, not here. Science is both a creative and collaborative field, and the community of scientists he was to be a part of (including Einstein) and eventually to lead, were all overseas. In particular, the Cavendish Lab at Cambridge had a legacy of science (and physics) breakthroughs. The infrastructure for his research was in place at Cambridge and also at McGill University where he also studied. There is pretty strong awareness, I believe, of Rutherford being one of our greatest achievers (he's usually paired with Edmund Hillary). Time magazine led with him on the cover of their millennium issue (South Pacific); there was the play at Circa last year, a new biography, a touring exhibition which has just opened at the Canterbury Museum, and of course the bank note, which I believe was influenced by public vote. I think Hillary is on the $5 note because it is among the most popular notes and Rutherford was on the "highest value" because of his Nobel achievement. Brian." Student Auckland, NZ